[ad_1]
By Lambert Strether of Corrente.
As readers know, I’m a big fan of anti-covid nasal sprays. The first time I did a round-up, in July of 2021, there were a few vaccines under development, but the focus was mainly on prophylaxis and treatment. As of this writing, there are many more solid nasal vaccine projects, worldwide, and one treatment on the market. So I’m encouraged!
I won’t go very deeply into the science behind the new generation of nasal vaccines (the previous post includes a diagram of the “human oral squamous epithelium” and how SARS-CoV-2 affects it). Rather, I will look at the advantages of nasal vaccines, ask how many nasal vaccines are under development (not so easy to answer), and look at a few of the projects in more detail. I’ll conclude with some brief caustic remarks on the Biden Administration’s miserably inadequate handling of this technology.
Advantages of Nasal Vaccines
Nasal vaccines have two significant advantages over current intramuscular (syringe-injected) vaccines.
First, the mjeans of administration means broader take-up.
Nasal vaccines don’t trigger fears of needles:
Trypanophobia is the extreme fear of injections or hypodermic needles. While it tends to be more common in children, this fear can remain well into adulthood for some people. Around 20% of adults in the world share this fear, with 1 in 6 adults avoiding the flu vaccine due to this phobia. If needles are keeping you from getting vaccinated against COVID-19, good news is on the way!
It’s always nice to have a word of the day!
Nasal vaccines are easier to administer:
Administration does not require a trained professional, sterile environment, and syringe and needle; making self-administration possible and thus offering much broader possibilities of distribution and a greater uptake in vaccination.
Self-administration is really attractive to me, since I avoid interacting with the health care establishment as much as I possibly can.
Some nasal vaccines can be easier to produce and store:
[Peter Palese, chairman of the Department of Microbiology at the Ichan School of Medicine at Mount Sinai Hospital] said one of the major advantages of [his] nasal vaccine is its ability to be stored in a normal fridge at 2-4 degrees Celsius, rather than the ultra-low temperatures required for the Pfizer and Moderna vaccines.
Because the vaccine is grown in chicken eggs — the same technology used for many flu vaccines all over the world — the cost of development is also cheaper. “It is much, much cheaper to produce this vaccine as compared to the mRNA vaccines by Pfizer and Moderna,” Palese told DW
Second, the mechanism of action promises sterilizing immunity. From the American Chemical Society:
SARS-CoV-2 often enters through our noses [because Covid is airborne], where it encounters a protein called ACE2, which is found in abundance in our nasal passages. ACE2 is the virus’s doorway into our cells. In fact, the mucosal membranes that line our airways, digestive systems, and reproductive tracts are often the first parts of our bodies to face an invading pathogen. A network of immune cells resides underneath our mucous membranes, or mucosae, and forms a front line of defense against invaders, and they prevent most infections from taking root. This is the mucosal immune system, and some immunologists think we have been seriously underestimating it.
“When you think about it, that’s where we acquire most of our infections: we inhale them, we consume them, or we get them through sex,” says Michael W. Russell, a mucosal immunologist and professor emeritus at the University at Buffalo.
Our mucosal immune cells make a special class of antibodies that are constantly secreted from the mucous membranes to protect the nose, gut, and other vulnerable sites from pathogens we’ve seen in the past. “But if you don’t stimulate the immune system in the mucosae, you don’t obtain mucosal immune responses,” says Pierre Charneau, head of the Molecular Virology and Vaccinology Unit at the Pasteur Institute.
Yet most research on SARS-CoV-2 and our immune systems has overlooked mucosal immunity in favor of the easier-to-study systemic immunity. “When the pandemic hit last year and I started to see papers coming out about immunity, it really quite staggered me to see an absence of attention to the mucosal immune response,” Russell says.
Charneau and a group of scientists in Paris have shown that natural SARS-CoV-2 infections trigger both systemic and mucosal immunity. But our current crop of COVID-19 vaccines offer only systemic protection. Developing vaccines that are sprayed up the nose, rather than injected into the arm, could change that, Charneau says.
Stopping infections where they start could mean sterilizing immunity. From the Lancet:
[Intranasal] vaccines have the potential to induce against mucosal pathogens. Antigens are exposed at the initial site of viral attack to induce potent immune responses at local or distant mucosa.
In a word, the [intranasal] immunization route can induce sterilizing mucosal and systemic immunity, further preventing virus infection and transmission. Application of [intranasal] vaccines will hopefully help deal with the persistent COVID-19 pandemic and potential viral contagious diseases in the future.
Time concludes:
[R]esearchers hope that nasal vaccines may one day do what even the highly effective mRNA vaccines made by Pfizer-BioNTech and Moderna have not: .
How Many Nasal Vaccines are Being Developed?
It’s not easy to determine how many nasal vaccines are in the works. I can’t browbeat Clinicaltrials.gov into giving me a figure less than 111, which is wrong. WHO says 9. The Lancet has a table that lists 12 (the figure used by the Times).
My guess is that the field is very dynamic, and the records-keeping hasn’t kept up. So let me just name a series of projects, so you can can see what I mean. One study, Altimmune, is known to have failed. Yale and Stanford have projects. Oragenics Inc. in Tampa, Florida and Inspirevax in Laval, Quebec have a hamster study. The Swiss National Fund has a phase 1 clinical trial, as do Sweden’s Karolinska institute, and Codagenix in the United States. France has a pre-clinical trial. Russia has a Sputnik spinoff. There are more projects in Mexico, at Washington University in St. Louis, and at Mount Sinai Hospital. I’m sure there are more; I could hardly turn around without stumbling over one. There are eleven mentioned here, plus three[2] I am about to list, which comes to fourteen, which is — allow me to take a moment and break out my calculator — two more than the twelve listed by the Lancet. Encouraging!
Examples of Nasal Vaccines
I want to delve more deeply into three projects in the United States, India, and Thailand.
First, the United States, at the National Institute of Allergy and Infectious Diseases (NIAID):
NIAID scientists have developed a candidate COVID-19 vaccine targeted for infants and young children that would require one dose delivered by a nasal spray.
In a new study published Dec. 7 in PNAS, NIAID’s Ursula Buchholz, Ph.D., Shirin Munir, Ph.D., Cyril Le Nouen, Ph.D., and colleagues in Bethesda, Maryland, and Hamilton, Montana, describe how they used a weakened version of a bovine/human parainfluenza virus (called B/HPIV3) to deliver SARS-CoV-2 spike protein to stimulate immunity against COVID-19. Think of how we use a grocery cart to carry food items to our vehicles. In this case, B/HPIV3 is the cart loaded with protective proteins that train our immune system to protect against SARS-CoV-2 and HPIV3. The vaccine is noteworthy because .
The group developed two versions of the vaccine for SARS-CoV-2 and compared them in hamsters to mimic COVID-19 in people. Candidate vaccine B/HPIV3/S-2P performed the best and was .
OK, granted, a hamster study. But this is the first I’ve heard of it, and I do try to keep track. What a shame the director of NIAID has no clout! Because otherwise they surely would have brought this excellent result before the public;
Second, India, at pharma giant Bharat:
Bharat Biotech, the maker of the first indigenous Covid-19 vaccine Covaxin, completed phase 1 trials on 400 healthy individuals.
In November last year, it completed phase 2 trials on about 650 volunteers in 10 centres across the country.
In the last week of January, the Drug Controller General of India (DCGI) permitted the vaccine maker to undertake phase 3 trials. BBV154 is an intranasal replication-deficient chimpanzee adenovirus SARS-CoV-2 vectored vaccine.
The vaccine has been developed using a new technology licensed from Washington University. Bharat Biotech believes in a cocktail approach of administering two doses of two different vaccines using a combination of intramuscular and nasal[3] in a first of its kind approach.
“If successful, intranasal vaccines make the roll out of booster doses easier and faster as administering intranasal vaccines is much easier when compared to that of intramuscular vaccines,” said one of the directors of a city-based corporate hospital adding that any danger of the fourth wave of the pandemic be dealt with a comprehensive programme for a booster dose to all.
The trials are expected to be complete in the second week of April.
Finally, Thailand:
A nasal spray said to be effective in preventing coronavirus infection is being developed by Thai scientists, and the product will be ready for launch later this year, according to the developers.
The spray is being developed under joint efforts by Chulalongkorn University, Silpakorn University, Health Systems Research Institute (HSRI), the Government Pharmaceutical Organization (GPO) and an undisclosed private bioscience company.
Dr Chanchai Sittipunt, dean of the Faculty of Medicine at Chulalongkorn University, said a cooperation agreement has been signed to produce the anti-Covid-19 nasal spray, and it could be a subject for global research.
Now the technology is being transferred to the private sector, which is conducting clinical research to meet the standards needed for registration with the country’s Food and Drug Administration, he said.
, adding Silpakorn University’s Faculty of Pharmacy has studied the spray with Chulalongkorn University’s Faculty of Medicine using lab animals.
This project is a little bit of a dark horse. Nevertheless, Chulalongkorn is a respected institution, and Thailand, which is facing the collapse of its tourism industry, has every incentive to get this right, not just for medical tourism, but all tourism.
Examples of Nasal Treatments
From the Economic Times of India:
Glenmark and its partner Canada-based SaNOtize Research & Development Corp. on Wednesday announced the launch of its Nitric Oxide Nasal Spray (NONS) under the brand name FabiSpray in India for the treatment of adult patients with COVID-19 who have high risk of progression of the disease.
NONS when sprayed over nasal mucosa acts as a physical and chemical barrier against the virus, preventing it from incubating and spreading to the lungs. Glenmark received manufacturing and marketing approval from India’s drug regulator for NONS as part of an accelerated approval process.
Glenmark said the Phase 3 trial in India met the key endpoints and demonstrated reduction of viral load of 94% in 24 hours and 99% in 48 hours, and was found to be safe and well tolerated in COVID-19 patients.
NONS has already received a CE mark in Europe, which is an equivalent of marketing authorization in case of a Medical Device.
By virtue of the CE mark, SaNOtize has permission to launch NONS in the EU.
NONS is also approved and being sold in Israel, Thailand, Indonesia and Bahrain, under the name enovid™ or VirX™.
So, world travelers, you can pick up a few boxes or a case of the stuff, eh?
Conclusion
One of the encouraging things about these nasal spray developments is they are either on the market already (NONS), or approaching the market (Bharat in India, and Thailand). Among the many, mahy heinous crimes of the Biden administration was not to accelerate the development of nasal spray technology. They had the example of the Other Guy’s Operation Warp Speed before them, and yet they sat on their hands, or diddled. It makes little difference, at this point, whether their indifference was from malice, or not. In either case, the endpoint was denial of life-saving medication to the citizenry they betrayed. It’s almost like they don’t want the pandemic to end.
NOTES
[1] Yes, but is that a sustainable business model?
[2] Bharat licenses Washington University’s technology, so perhaps my definition of “project” doesn’t line up with the Lancets. Ah well, nevertheless.
[3] Wrong. This is Yale’s approach.
APPENDIX 1
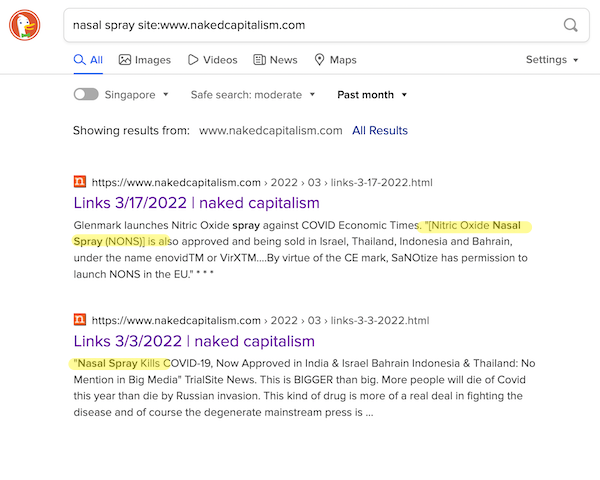
Here is Google:
No hits, but the ad is a very nice touch.
Here are the results I was looking for, because I knew they existed:
It’s pretty hard to argue this isn’t outright censorship from Google. Badge of honor, so far as I’m concerned.
APPENDIX 2
Speaking of nasal sprays as prophylactics, the FTC isn’t happy with Povidone. But the Povidone market is expected to do very well.
[ad_2]
Source link