[ad_1]
In 2021, the FDA’s Center for Drug Evaluation and Research (CDER) approved 50 new drugs that are either New Molecular Entity (NME) under New Drug Application (NDA) or Biologics License Application (BLA) New therapeutic biologics under the FDA. The FDA summarized these approvals in its recently released report, titled: Promoting Health Through Innovation: New Drug Treatment Approvals in 2021.
Based on recent trends, 2021 can be seen as a year of above-average innovation. Between 2012 and 2020, the average number of approved drugs was 42.2 (median: 45). Therefore, we can view 2021 as an above-average year. This is especially true given the potential slowdown in clinical trials caused by COVID-19.
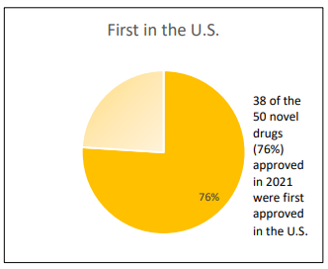
Interestingly, 76% (n=38/50) of new drugs approved in 2021 were approved in the US earlier than any other country. Part of the reason is the speed of the FDA relative to the rest of the world’s regulators. FDA reports that 86% of approved drugs are approved within their first cycle; FDA claims this fact “reflects the extent to which CDER staff provide drug developers with necessary study design elements and drug applications” Additional data required to support a comprehensive and comprehensive drug evaluation.” However, part of the reason so many new drugs are on the market in the U.S. is the strategic decision by pharmaceutical companies to file for approval in the U.S., the world’s largest pharmaceutical market.
We can break down approvals for many other characteristics.
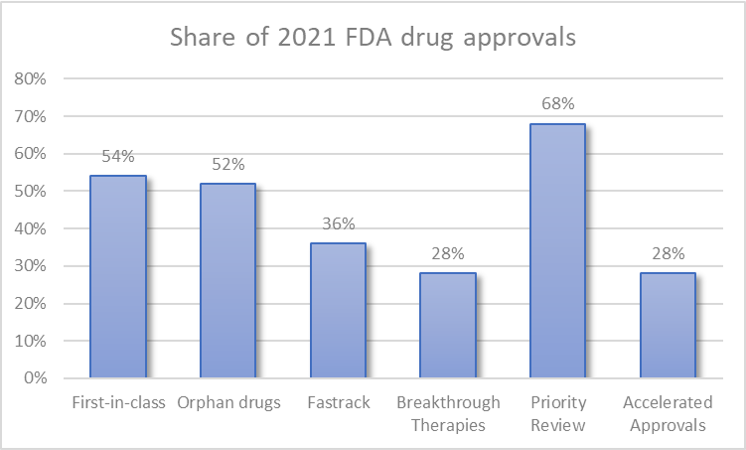
People may be concerned that many drugs are approved as “me too”. However, we saw that more than half (54%, n=27/50) of approvals were for first-in-class drugs. These approvals include (Adbry, Aduhelm, Besremi, Brexafemme, Bylvay, Cosela, Cytalux, Empaveli, Evkeeza, Kerendia, Korsuva, Leqvio, Livtencity, Lumakras, Lupkynis, Nulibry, Rezurock, Rybrevant, Saphnelo, Tavneos, Tezspire, Tivdak, Verquvo, Voxzogo, Vyvgart, Welireg, Zynlonta). Interestingly, 52% of approvals (n=26/50) were for orphan drug indications.
More details on approved names are below.
- Fast Track (36%). Fast Track accelerates the development and review of new drugs by improving the level of communication between FDA and drug developers and by enabling CDER to review portions of drug applications on a rolling basis. Drugs include Aduhelm, Amondys 45, Brexafemme, Bylvay, Cabenuva, Cytalux, Empaveli, Exkivity, Kerendia, Lumakras, Lupkynis, Nexviazyme, Rylaze, Saphnelo, Scemblix, Truseltiq, Verquvo, Vyvgart
- Breakthrough Therapy (28%). Breakthrough Therapy designation includes all Fast Track program features and provides the FDA with more in-depth drug development efficiency guidance. Relevant drugs include Cosela, Evkeeza, Exkivity, Jemperli, Korsuva, Livmarli, Livtencity, Lumakras, Nexviazyme, Nulibry, Rezurock, Rybrevant, Scemblix, Ukoniq.
- Priority review (68%). If CDER determines that the drug is likely to make significant advances in health care, the drug will receive priority review. This means that CDER aims to take action on drug applications within six months of submission (compared to 10 months for standard review).
- Accelerated approval (28%)The accelerated approval pathway gives the FDA more flexibility on which endpoints it can use to approve a drug that is more beneficial than current treatments for serious or life-threatening conditions. These accelerated approval endpoints may include those that are “likely” to predict clinical benefit, which may allow the drug to show benefit over a shorter treatment period (whereas traditional approval requires longer-term proof of benefit). Subsequent confirmatory testing must be conducted to support traditional approval. The program is designed to bring drugs that offer important therapeutic advances to market faster than traditional approvals. Drugs approved under this pathway include: Aduhelm, Amondys 45, Exkivity, Jemperli, Lumakras, Pepaxto, Rybrevant, Scemblix, Tepmetko, Tivdak, Truseltiq, Ukoniq, Voxzogo, Zynlonta.
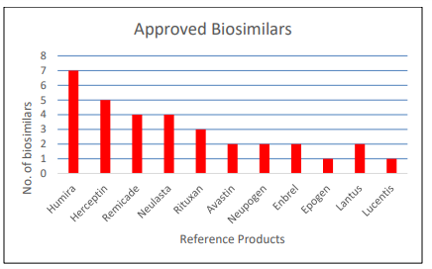
The FDA also approved 4 new biosimilar products in 2021. Since 2015, the FDA has approved 33 biosimilar products for 11 different reference biotherapeutics.
[ad_2]
Source link








